BioNJ, New Jersey’s life sciences trade association, hosted the Inaugural Health Equity in Clinical Trials MBA Business Plan Case Competition on Saturday, December 3, at Rutgers Business School. BioNJ’s Business Plan Case Competition, which is part of a broader BioNJ strategic initiative of Health Equity in Clinical Trials, was designed to promote the next generation of diverse clinical trial innovators and identify innovative approaches and successful models that can be used nationally to strengthen diversity in clinical trials and expand health equity.
Eight teams, competing for more than $20,000 in prize money, were tasked with developing a business plan defining a new solution, application or technology to help address this important and challenging problem. Teams identified one particular type of health disparity on which to frame their proposed solutions and connected with community-based organizations to better understand the real-world barriers that exist for their chosen populations to engage in clinical trials. The disease areas on which the respective teams focused included Alzheimer’s, heart failure, multiple sclerosis, diabetes type 1 and type 2, colorectal and cervical cancers, and oncology.
The 2022 winning teams:
- First Place: Johns Hopkins University ($10,000) who focused their plan on increasing representation in clinical trials for the treatment of Alzheimer’s Disease through community engagement strategies and the use of digital tools.
- Second Place: Rutgers University ($7,000) who focused their plan on using population health and real-world data analytics to calculate health equity targets specific to clinical trial sites in prostate cancer.
- Third Place: Baylor College of Medicine, Northwestern University & Rice University ($3,500) who focused their plan on increasing Hispanic/Latinx involvement in clinical trials by training community healthcare workers from refugee and immigrant populations.
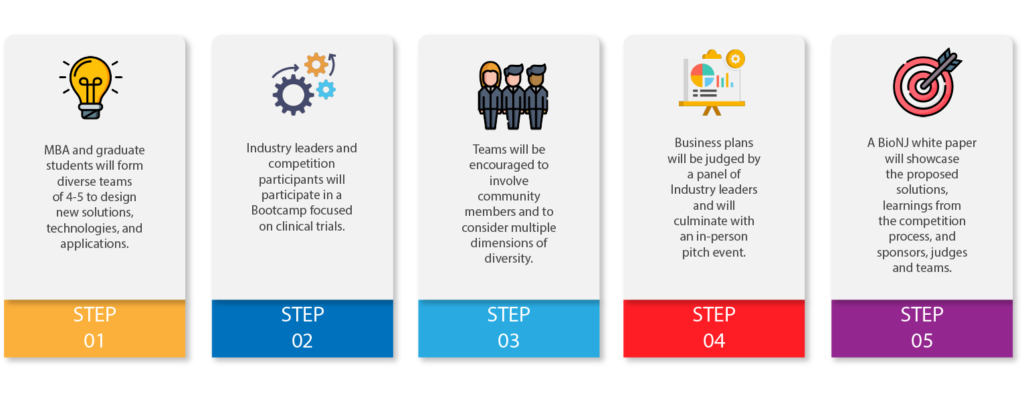
Details from the 2022 Competition
Prizes and Other Benefits
- Make a difference and gain expertise in health equity, clinical trials, and medical product development.
- The first, second and third place teams are eligible for a cash award
- → 1st – $10,000
- → 2nd – $7,000
- → 3rd – $3,500
- Teams will have their resume book shared with competition sponsors and judges.
- Teams will have opportunities to network with life sciences industry professionals.
- Teams and proposals will be highlighted in a broadly distributed white paper.
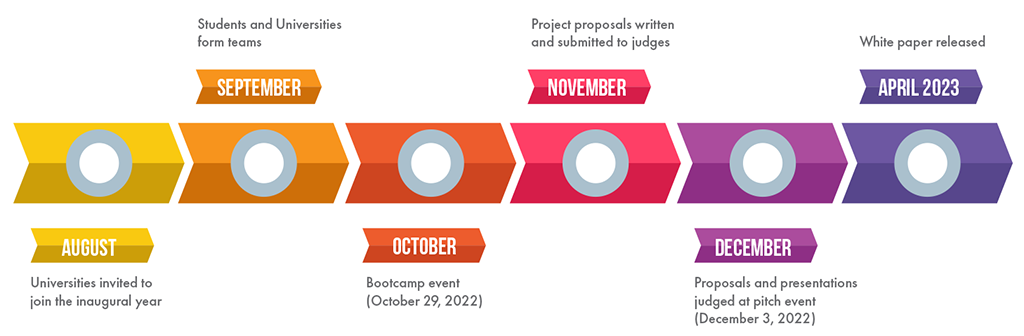
Timeline
Team Requirements
- Students from invited MBA programs should self-organize a diverse 4-5 person team to represent the university, drawing on guidance from peers, faculty, staff, and other graduate programs. Each team must include at least one MBA student.
- In the case of multiple teams registering from the same university, the project steering committee will review team credentials and make a final selection for participation in the competition.
- Teams should be multidisciplinary and can include at least one MBA as well as graduate students from different degree-seeking programs (such as MD, PharmD, MPH, etc.)
- Teams can work virtually or face-to-face.
- Proposals must be product agnostic.
- Teams will be encouraged to seek input from local community organizations.
- If your school does not have travel funds available, please contact us to discuss travel assistance.
Project Topics
- Teams will receive a crash course in medical product development and clinical trials at the October Bootcamp event.
- Proposals can be new solutions, applications, or technologies on topics such as using artificial intelligence, increasing access, integrating with care delivery, and furthering decentralized clinical trials.
- Ideas should be scalable.
- Proposals can focus on varying elements of racial and ethnic diversity. They can also focus on other underrepresented populations defined by demographics such as sex, gender, identity, age, socioeconomic status, disability, pregnancy status, lactation status, and co-morbidity. (For more, see the FDA Guidance on Diversity Plans to Improve Enrollment in Clinical Trials.)
Competition Format & Logistics
An in-person December 3, 2022 pitch event will position your team’s business proposal with industry leaders. All teams and proposals will be highlighted in a widely circulated white paper following the competition, earning valuable visibility for you, your school and your proposed solution to this business-critical challenge.
 | Download BioNJ’s Health Equity in Clinical Trials MBA Business Plan Competition Rules & Requirements here. |
 | Download BioNJ’s Health Equity in Clinical Trials MBA Business Plan Cover Sheet here. |
Location:
Rutgers Business School
100 Rockafeller Road
Piscataway, NJ 08854
Competition Judges
Bootcamp Format and Logistics
On October 29, 2022, BioNJ held a virtual Bootcamp event to provide a crash course on drug development and pain points that lead to racial, gender, disability and other disparities in clinical trial participation. Registered teams had the opportunity to meet, hear from, and pose questions to expert advisors.
Topics for discussion included:
- Drug development and clinical trials 101
- Review the competition process and evaluation criteria
- Orient student teams to clinical trials and the role of clinical trials in therapy development
- Outline the current inequities within clinical trials
- Frame the challenges that stem from lack of representation in clinical trials
- Introduce key resources to inform proposals
Resources & Suggested Reading
Pain Points and Challenges
An Introduction to Medical Product Development
Success Factors
Economic Impact of Medical Research
- BioNJ – Economic Impact Study of Clinical Trials Activity in New Jersey
- EveryLife Foundation – National Economic Burden of Rare Disease Study
- Tufts Center for the Study of Drug Development – Innovation in the pharmaceutical industry: New estimates of R&D costs
About Medical Product Development and Clinical Trials
- CISCRP – Finding Treatments Together series
- National Library of Medicine – ClinicalTrials.gov (beta site)
- Craig Lipset – Decentralized Clinical Trials: Collaboration to Accelerate Adoption
- Food and Drug Law Institute – Considering Modifications to Existing FDA Regulatory Incentives to Achieve Greater Racial and Ethnic Diversity in Pivotal Clinical Trials for Drug Approvals
- George Washington University – The Value of Clinical Trials
- PhRMA – The Biopharmaceutical Research and Development Process
- U.S. Food and Drug Administration – Patient-Focused Drug Development
About Health Equity
- Clinical Pharmacology & Therapeutics – Roadmap to 2030 for Drug Evaluation in Older Adults
- Crossroads4Hope – Diversity Matters, Advancing Cancer Treatments Through Increased Clinical Trial Participation
- Endpoints News – CMS as gatekeeper: Why lecanemab is similar but also very different from Aduhelm
- Multi-Regional Clinical Trials – Equity by Design in Clinical Research: The EbD Metrics Framework
- National Academies of Sciences, Engineering, and Medicine – Improving Representation in Clinical Trials and Research
- Companion article that emphasizes economic impact and model used
- Companion webinar (1 hour)
- National Equity Atlas
- New York Times – In Cancer Trials, Minorities Face Extra Hurdles
FAQs
How do we get started?
Once you and your team of 4-5 students (at least one from your school’s MBA program) are registered as a team, review the documents in the “Case and Proposal Components” section of the website. Pay close attention to the “Competition prompt/case statement.”
When considering the focus of your proposal, refer to the resources that we have created and curated in the “Resources & Suggested Reading” section of the website.
If you are new to health care and medical research, you might find our background videos particularly helpful: Bringing A New Treatment to Market: An Introduction to Medical Product Development and Representative Clinical Trials: Pain Points and Challenges.
What should our proposals include?
Review the Competition Rules & Requirements (available in the “Case and Proposal Components” section of the website). Your submission has 2 parts:
- Written proposal (up to 8 pages) plus the cover page and up to 5 additional pages for references, appendices, background material. This is due by 5:00 PM (ET) on November 28, 2022.
- Presentation deck that you will use at the live pitch event to describe your proposal. This is due by 5:00 PM (ET) on December 1, 2022. You will have 7 minutes for your pitch. You may want to have additional slides for back-up information that you can draw on in responding to judges’ questions. No changes to the deck can be made after you submit it.
Be sure to watch our Competition Success Factors video, which walks you through everything you need to know.
Should our proposal cover all of the potential angles of the problem we’re looking to address? (For example, policy, regulatory, community, scientific implications?)
Definitely not. The issue of lack of representative clinical trials is a big one that cannot be solved with one solution. We’re asking you to focus on one solvable piece of the complex puzzle. You’ll likely need to make certain assumptions within your proposal, which is absolutely fine.
Should our proposal be US-focused? Or global?
For this inaugural year, your proposal should be US-focused.
Can we get expert advice on our proposals?
Yes! You’re welcome to seek advice from faculty, staff, peers, community members, etc. that you know. We also have industry experts that can answer your questions. Send your questions to sam@kithcollective.com, and we will facilitate getting you a response. We will curate general questions/responses (ones that aren’t proposal-specific) and share them with all registered teams, so that all Competition participants have access to the same information.
Can I get a recording of the Bootcamp from October 29?
No problem. Reach out to sam@kithcollective.com to request the link.
What are some examples of community groups we should talk to?
You can engage a wide variety of community-based groups or organizations. Take a look at the affiliate groups that are part of your University and would be relevant to your proposal’s topic or intended population. You might also be able to find a local chapter or affiliate of a national organization. Think about community health centers, youth and/or senior centers, and faith-based organizations that host groups, too.
What are the due dates?
Submit your proposal (following the format outlined in the Competition Rules & Requirements) to sam@kithcollective.com by 5:00 PM ET on November 28, 2022, as a PDF or Word document.
Submit your presentation to sam@kithcollective.com by 5:00 PM ET on Thursday, December 1, 2022, as a PowerPoint document. (Also, save it on a flash drive that you can bring with you to the Competition pitch event!)
When and where is the competition?
The Competition pitch event is taking place in-person on Saturday, December 3 from 10 a.m. until 4:30 p.m. ET at Rutgers University. The address is Rutgers Business School, 100 Rockafeller Road, Piscataway, NJ 08854.
How should we get there and where should we stay?
The latest details about travel and transportation can be found on our website. Please reach to sam@kithcollective.com with any specific questions or concerns.
Can you help with travel expenses?
Students will be responsible for booking their travel. Student teams should consult with their university for travel authorization and/or reimbursements. If a school or team requires additional travel assistance funds, please reach out to sam@kithcollective.com to inquire about available funds.
Competition Contact
Samantha Mayberry
Director of Client Services,
Kith Collective
sam@kithcollective.com











































